Science 27 September 2013: Vol. 341 no. 6153 pp. 1502-1505. DOI: 10.1126/science.1241488
Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide
Maria R. Lukatskaya1,2, Olha Mashtalir1,2,*, Chang E. Ren1,2,*, Yohan Dall’Agnese1,2,3,4, Patrick Rozier3, Pierre Louis Taberna3, Michael Naguib1,2, Patrice Simon3,4, Michel W. Barsoum1, Yury Gogotsi1,2
 1Department of Materials Science and Engineering, Drexel University, Philadelphia, PA 19104, USA.
1Department of Materials Science and Engineering, Drexel University, Philadelphia, PA 19104, USA.
2A. J. Drexel Nanotechnology Institute, Drexel University, Philadelphia, PA 19104, USA.
3Université Paul Sabatier, CIRIMAT UMR CNRS 5085, 118 route de Narbonne, 31062 Toulouse, France.
4Réseau sur le Stockage Electrochimique de l’Energie (RS2E), FR CNRS 3459, France.
Abstract
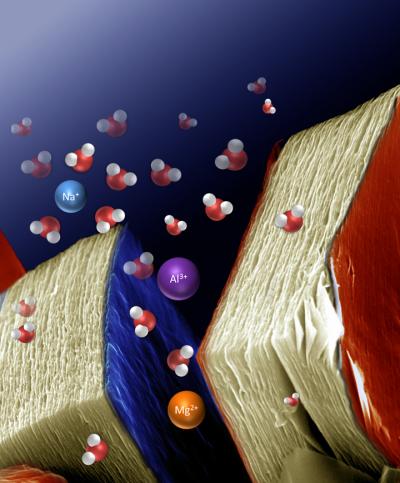
Many batteries and capacitors make use of lithium intercalation as a means of storing and transporting charge. Lithium is commonly used because it offers the best energy density, but also because there are difficulties in storing larger cations without disrupting the crystal structure of the host. Lukatskaya et al. developed a series of MX compounds, where M represents a transition metal and X is carbon or nitrogen.The compound Ti3C2 forms a two dimensional layered structure, which is capable of accommodating a wide range of cations, including multivalent ones, either spontaneously or electrochemically.
READ MORE on www.mrc.org.ua