J.K. McDonough, Y. Gogotsi, Carbon Onions: Synthesis and Electrochemical Applications, Interface, Fall 2013, 61-66 (2013)
Beginning with fullerenes, moving to carbon nanotubes, and most recently to graphene, carbon nanomaterials are widely studied and used in a range of applications including electronics, tribology, and energy storage. However, two kinds of carbon nanoparticles, nanodiamond1 and carbon onions, which were discovered before fullerenes and nanotubes, stayed for a long time in the shadow of more popular and better investigated nanocarbons. However, both have become increasingly studied in recent years. Carbon onions consist of spherical closed carbon shells and owe their name to the concentric layered structure resembling that of an onion. Carbon onions are sometimes called carbon nano-onions (CNOs) or onion-like carbon (OLC). Those names cover all kinds of concentric shells, from nested fullerenes to small (<100 nm) polyhedral nanostructures. This review is dedicated to those materials. We first discuss the structure of carbon onions and provide an overview of their synthesis methods. Also, electrochemical applications of carbon onions are reviewed with an emphasis on supercapacitor electrodes.
Sumio Iijima discovered OLC in 1980 while looking at a sample of carbon black in a transmission electron microscope. OLC was not produced in bulk, but rather was observed as a byproduct of carbon black synthesis. About a decade later in 1992, Daniel Ugarte put forth a formation mechanism for the spherical graphitic structure. By focusing an electron beam on a sample of amorphous carbon, he was able to observe the formation of OLC in situ. Under an electron beam, the amorphous carbon graphitizes and begins to curl, and after sufficient time, the graphitic carbon closes on itself, forming an onion. The curving and closure occurs in order to minimize the surface energy of the newly formed edge planes of graphite, which is about 30x that of the basal plane.
Synthesis of Carbon Onions
Although OLC has been synthesized by many different methods in the last 30 years, large scale production (gram quantities) of OLC was first realized in 1994 by Vladimir Kuznetsov and co- workers, who used vacuum annealing of a nanodiamond precursor. 5,6 Similar to vacuum annealing, other groups have also utilized annealing in inert gases to transform nanodiamond, which is currently produced in ton quantities, 1 to OLC. 7 This is one of the methods that has a potential for industrial applications, as the onion yield is close to 100% and the manufacturing volume is only limited by the size of the furnace, and can be scaled accordingly. This material rarely has ideal spherical carbon onions, but can be produced in large quantities and finds practical applications. The transition of nanodiamond to a carbon onion can be seen in a molecular dynamics (MD) simulation (Fig. 1a-c). A 2-nm particle of nanodiamond (Fig. 1a) was annealed at 1400 °C (Fig. 1b) causing the outer layers of the nanodiamond to convert to graphitic carbon; however the annealing was not at high enough temperature to convert the entire particle. At higher temperatures (Fig. 1c), the entire particle is converted to an OLC particle. 8 At the highest annealing temperatures, the OLC particle begins to polygonize (Fig. 1d) as the structure becomes more ordered. The particle size of OLC produced via nanodiamond annealing is dependent on the nanodiamond precursor, which is generally about 5 nm in diameter, 1 producing onions in the 5-10 nm size range.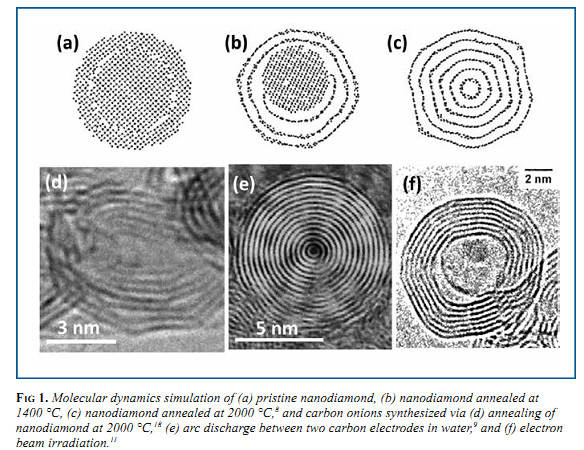
Arc discharge between two graphite electrodes in water represents another synthesis technique, generating OLC of slightly different structure than from annealing of nanodiamond. A dc current of 30 A and 17 V was applied between two graphite electrodes in water causing the carbon to evaporate at the location of the arc due to the extreme heat generated. The carbon vapor rapidly condenses into highly spherical OLC particles (Fig. 1e) and will float on the water surface, waiting to be collected for analysis. Consumption of the anode was about 100 mg/min, with the carbon products being produced at 20 mg/min. Synthesis by arc discharge can be performed at ambient pressure and temperature, avoiding the use of expensive equipment or catalysts, however the yield is low and samples contain nanotubes and amorphous carbon formed along with carbon onions.
Read more about carbon onions research