|
Where Do Batteries End and Supercapacitors Begin? |
The different mechanisms of capacitive energy storage are illustrated...
Electrochemical measurements can distinguish between different types of energy storage materials and their underlying mechanisms.
Batteries keep our devices working throughout the day — that is, they have a high energy density—but they can take hours to recharge when they run down. For rapid power delivery and recharging (i.e., high power density), electrochemical capacitors known as supercapacitors are used. One such application is regenerative braking, used to recover power in cars and electric mass transit vehicles that would otherwise lose braking energy as heat. However, supercapacitors have low energy density. Batteries and supercapacitors both rely on electrochemical processes, although separate electrochemical mechanisms determine their relative energy and power density.
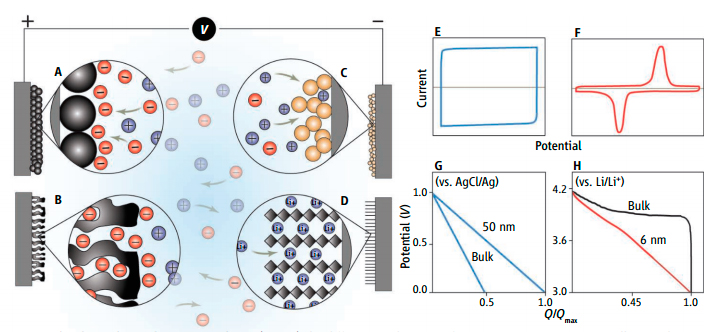
During the past 5 to 7 years, the energy storage field has witnessed a dramatic expansion in research directed at materials that might combine the high energy density of batteries with the long cycle life and short charging times of supercapacitors. However, the blurring of these two electrochemical approaches can cause confusion and may lead to unwarranted claims unless careful attention is paid to fun-damental performance characteristics. Read More about Batteries and Supercapacitors |